What Is Verifiedenal’s Compliance Check?
A Compliance Check is a regulatory and documentation review process used to verify whether a medical or dental device manufacturer meets export-market requirements before production or shipment begins.
Verifiedental’s Compliance Check emphasizes real-world regulatory readiness rather than surface-level paperwork. The review covers device classification pathways, ISO 13485 quality systems, FDA and CE documentation routes, technical files, labeling and packaging conformity, supplier declarations, and export eligibility—allowing overseas buyers to proceed with sourcing decisions with regulatory confidence.
This service helps brands reduce the risk of customs holds, shipment delays, certification failures, and post-market enforcement actions caused by missing filings, inaccurate declarations, or misunderstood compliance obligations.
We Verify Every Layer of Your Documentation.

CLEAR RESULTS
We Translate “Legal Jargon” into “Yes or No”
You don’t need a law degree to understand our report. We don’t send you a confusing 50-page regulation document. We give you a clear, actionable answer within 24 hours: Is the certificate valid? Does it cover your product? Is it safe to ship? We highlight the risks in red so you can make a quick decision.
Why Choose Verifiedental Compliance Check Service?
We Don’t Guess. We Verify.
Most delays happen because of mismatched paperwork.We combine digital verification with physical inspectionsto ensure your documents and your cargo tell the exactsame story.
24-Hour Turnaround
Compliance checks shouldn’t delay your shipment. We verify certificates and issue a ‘Green Light’ report within 24 hours so you can book your vessel on time.
Zero “Photoshop” Risk
We never rely on the PDF file sent by the supplier. We validate directly with the issuing body’s official database (TUV, SGS, FDA) to detect even the best forgeries.
Physical Consistency
A valid certificate is useless if the product label doesn’t match it. We physically check the cargo to ensure the Model No. on the box matches the CE certificate exactly.
Avoid Customs Seizures
Our checklist is based on actual US/EU customs seizure data. We identify ‘Red Flag’ keywords and labeling errors that trigger inspections, saving you thousands in fines.

SCENARIO A:THE HIDDEN SUSPENSION
The FDA number lookedvalid, but the status was”Inactive”
A supplier might send you a real registration number. Butwhat they don’t tell you is that it was suspended lastmonth due to late payments or compliance issues.
How We Fix It: We don’t just read the PDF. We query the live FDA/NMPA database to verify the current legal status ofthe manufacturer.
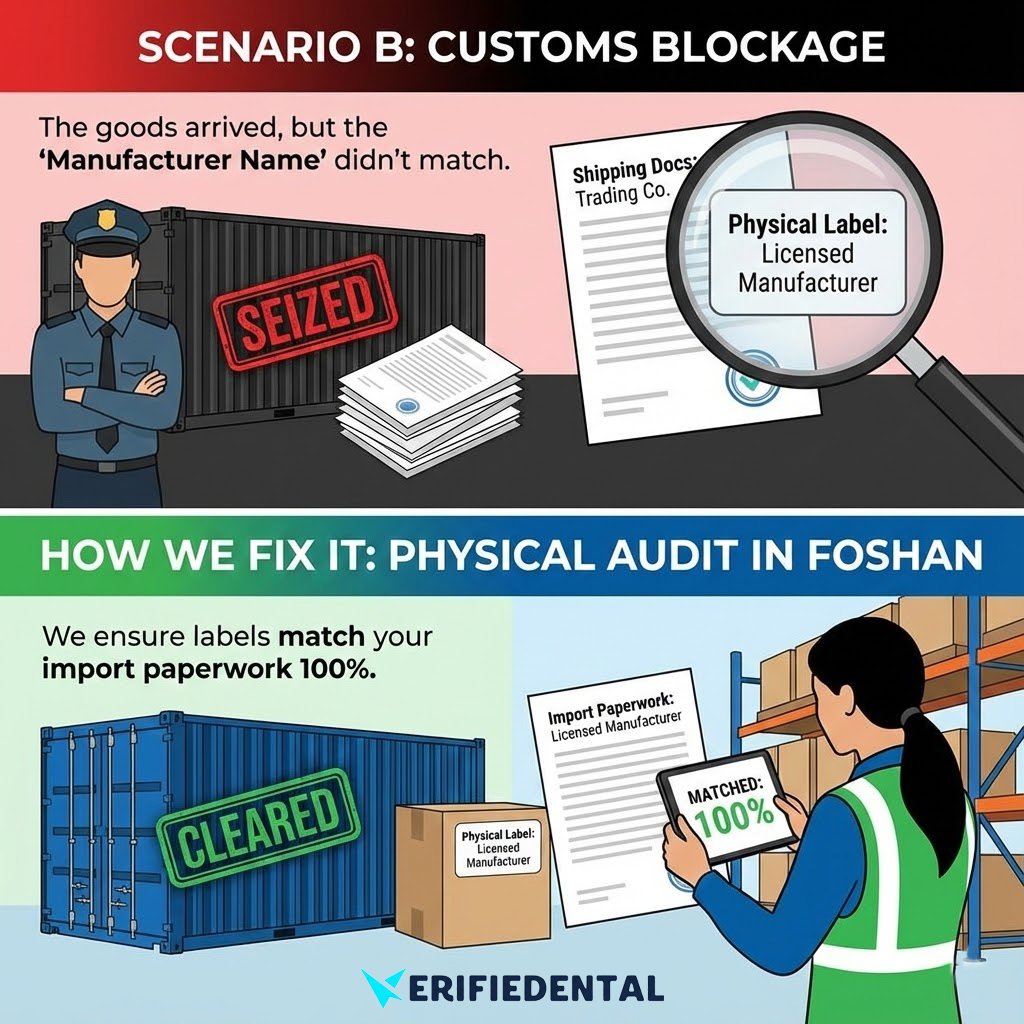
SCENARIO B:CUSTOMS BLOCKAGE
The goods arrived, but the”Manufacturer Name”didn’t match.
Customs officers strictly compare the shipping documents with the physical product label. A simple typoor using a ‘Trading Company’ name instead of the’Licensed Manufacturer’ can cause your container to beseized.
How We Fix It: We physically audit the labels in Foshan to ensure they match your import paperwork 100%.

